Note To File Template
Note To File Template - Protocol number, version and date:. Be signed and dated by the. A note to file is a way to document deviations, problems, or events during human subjects research that cannot be recorded in other forms. 2.1 all notes to the study file should be signed by the author, kept on file in the site regulatory file and made available to the clinical site monitors reviewing the site’s documents and. A note to file (ntf) may be used to: A template and examples are. If the issue relates to site performance, the appropriate credentialed individual from the site should write and sign the note to file. The purpose for this sop is to define the reason for crafting a note to file (ntf) and provide a note to study file template Ote to file (ntf)] clinical research site. Find templates and guidance for developing and conducting clinical research protocols, informed consent materials, and regulatory documents. Download a template to document events or issues in a research study. Protocol number, version and date:. A note to file (ntf) may be used to: Download a template for a standard note to file format and see examples of deviations, problems, and events to document. 2.1 all notes to the study file should be signed by the author, kept on file in the site regulatory file and made available to the clinical site monitors reviewing the site’s documents and. The purpose for this sop is to define the reason for crafting a note to file (ntf) and provide a note to study file template Ote to file (ntf)] clinical research site. This file provides a template for creating a note to file, including specific guidelines and a sample note. This template is provided by the office for the protection of research subjects (oprs) at the university of illinois. This web page provides a description of. Protocol number, version and date:. Download a template to document events or issues in a research study. This file provides a template for creating a note to file, including specific guidelines and a sample note. See a sample template and tips for clarity, conciseness, and signatures. Dmid notes to the study file guideline & template 9 july 2021 page 2. It outlines when and how this documentation should be used. The study management templates are a university of michigan resource available to all study team members. Issuu turns pdfs and other files into interactive flipbooks and engaging content for every channel. Principal investigator/ investigator of record: A template and examples are. Download a template for a standard note to file format and see examples of deviations, problems, and events to document. Include the subject and protocol to which it refers. Principal investigator/ investigator of record: Ote to file (ntf)] clinical research site. The purpose for this sop is to define the reason for crafting a note to file (ntf) and provide. A note to file (ntf) may be used to: It outlines when and how this documentation should be used. Download a template for a standard note to file format and see examples of deviations, problems, and events to document. See a sample template and tips for clarity, conciseness, and signatures. Ote to file (ntf)] clinical research site. 2.1 all notes to the study file should be signed by the author, kept on file in the site regulatory file and made available to the clinical site monitors reviewing the site’s documents and. Issuu turns pdfs and other files into interactive flipbooks and engaging content for every channel. [insert date of the ; • explain a discrepancy, the action. Learn what a note to file is, when to use it, and how to write one. 2.1 all notes to the study file should be signed by the author, kept on file in the site regulatory file and made available to the clinical site monitors reviewing the site’s documents and. A template and examples are. This template is provided by. A template and examples are. Issuu turns pdfs and other files into interactive flipbooks and engaging content for every channel. Find templates and guidance for developing and conducting clinical research protocols, informed consent materials, and regulatory documents. Be signed and dated by the. This file provides a template for creating a note to file, including specific guidelines and a sample. 2.1 all notes to the study file should be signed by the author, kept on file in the site regulatory file and made available to the clinical site monitors reviewing the site’s documents and. See a sample template and tips for clarity, conciseness, and signatures. [insert date of the ; If the issue relates to site performance, the appropriate credentialed. The purpose for this sop is to define the reason for crafting a note to file (ntf) and provide a note to study file template Ote to file (ntf)] clinical research site. Principal investigator/ investigator of record: Download a template to document events or issues in a research study. If the issue relates to pi responsibilities (e.g., human subject. A template and examples are. [insert date of the ; Download a template to document events or issues in a research study. 2.1 all notes to the study file should be signed by the author, kept on file in the site regulatory file and made available to the clinical site monitors reviewing the site’s documents and. • explain a discrepancy,. • explain a discrepancy, the action taken in. A note to file (ntf) may be used to: Download a template to document events or issues in a research study. Dmid notes to the study file guideline & template 9 july 2021 page 2 of 3 format and content the ntf should be written on institutional letterhead and include the following elements. The templates are optional and can be customized to suit study team needs. 2.1 all notes to the study file should be signed by the author, kept on file in the site regulatory file and made available to the clinical site monitors reviewing the site’s documents and. • explain the location of a study document when it is not filed in the expected location; This document provides guidance on how to create a note to file for human subjects research. Download a template for a standard note to file format and see examples of deviations, problems, and events to document. This template is provided by the office for the protection of research subjects (oprs) at the university of illinois. It ensures compliance with documentation standards. The purpose for this sop is to define the reason for crafting a note to file (ntf) and provide a note to study file template If the issue relates to site performance, the appropriate credentialed individual from the site should write and sign the note to file. If the issue relates to pi responsibilities (e.g., human subject. This file provides a template for creating a note to file, including specific guidelines and a sample note. Find templates and guidance for developing and conducting clinical research protocols, informed consent materials, and regulatory documents.Note To File Template Clinical Research
Legal File Note Law Client Note Template Meeting Notes Telephone Note
Microsoft Word Note Taking Template For Your Needs
Meeting Notes Template 30+ Word, PDF Documents Download
EXCEL of Simple Notes Description.xlsx WPS Free Templates
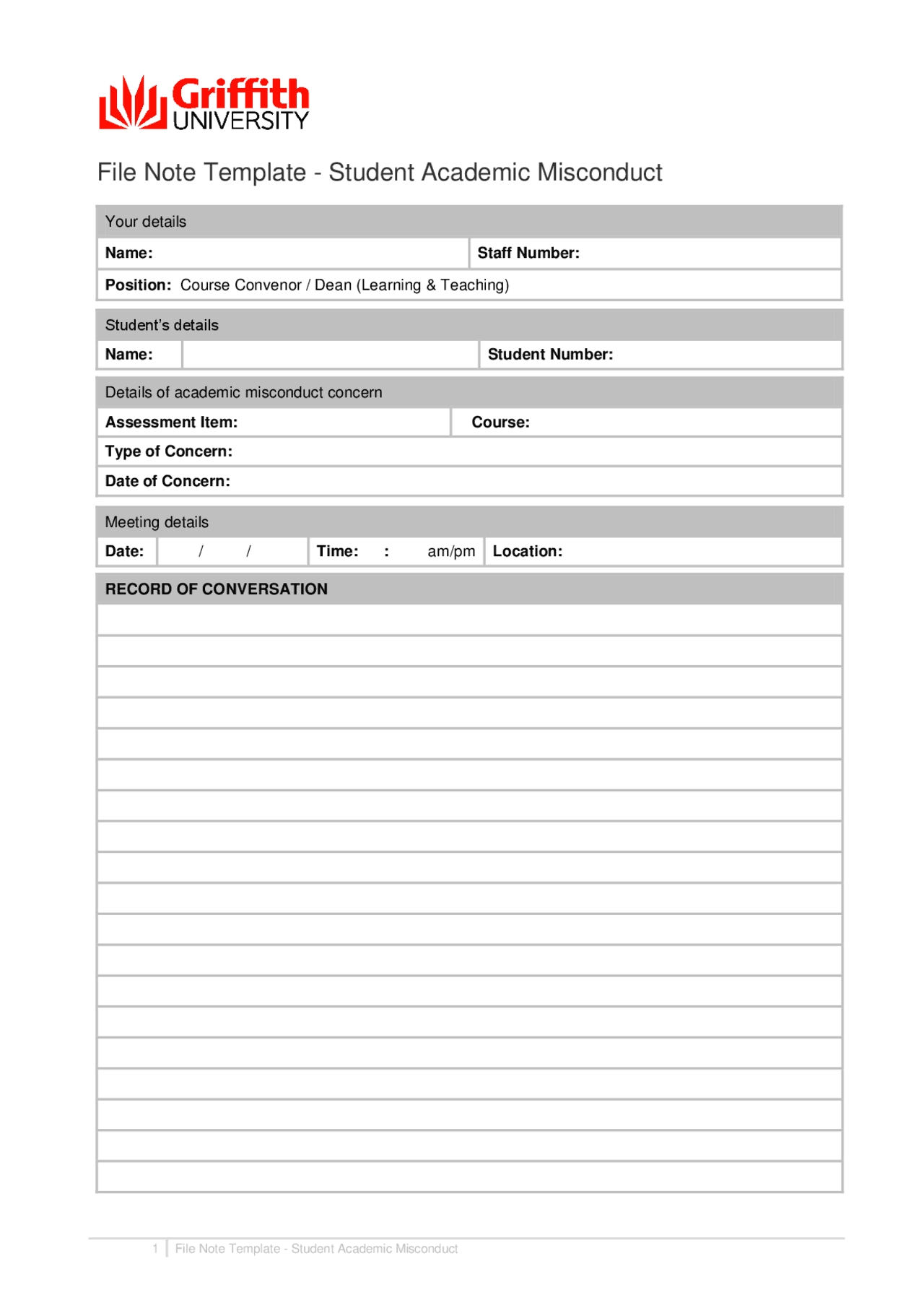
File Note Template Student Academic Misconduct Study Guides
Note Taking Template Word in 2022 Cornell notes template, Notes
NoteToFile Template
Legal File Note Template Notes template, Feedback for students, Book
Note To File Template Download by Pharma Student Issuu
Ote To File (Ntf)] Clinical Research Site.
A Note To File Is A Way To Document Deviations, Problems, Or Events During Human Subjects Research That Cannot Be Recorded In Other Forms.
The Study Management Templates Are A University Of Michigan Resource Available To All Study Team Members.
Learn What A Note To File Is, When To Use It, And How To Write One.
Related Post: