Medical Clinical Trial Protocol Template
Medical Clinical Trial Protocol Template - The interventional drug/device trial template and the behavioral and social science research template both. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human participants. Fgs provided statistical expertise in clinical trial design. Cfaam, bgp, fgs, dsmp, and pmr conceived the idea and study design. Prospective data and/or sample collection 3. However, others may also find this template. Secukinumab (ain457) clinical trial protocol cain457a2311 / nct03668613. Welcome to global health trials' tools and templates library. Phase 2 or 3 clinical trials that require. After reading, you will understand how to find a relevant clinical. Background medical students face highly competitive stressful situations throughout their curriculum, which can lead to elevated stress levels and a major decline in quality of life,. Its use will also help. Investigators for such trials are strongly encouraged to use this template when developing protocols for nih supported clinical trial(s). The protocol is the backbone of your clinical trial, detailing every step of the study. Fgs provided statistical expertise in clinical trial design. Welcome to global health trials' tools and templates library. Clinical trials conducted after regulatory submission of a dossier, but prior to the medicine's approval and launch. Ance for industry, e6 good clinical practice: In this blog, you have access to the links to the clinical trial protocol template from several regulatory bodies. Please use this template for the following study types: There are two templates to be used for interventional research: Welcome to global health trials' tools and templates library. Clinical trials conducted after regulatory submission of a dossier, but prior to the medicine's approval and launch. Phase 2 or 3 clinical trials that require. Secukinumab (ain457) clinical trial protocol cain457a2311 / nct03668613. Prospective data and/or sample collection 3. These trials may supplement earlier trials, complete earlier trials, or may. The interventional drug/device trial template and the behavioral and social science research template both. The protocol is the backbone of your clinical trial, detailing every step of the study. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Clinical trial protocol cqge031c2303 / nct03580356. Its use will also help. Cfaam wrote the draft version. Ance for industry, e6 good clinical practice: Clinical trial protocol cqge031c2303 / nct03580356. The protocol is the backbone of your clinical trial, detailing every step of the study. It ensures consistency across clinical trial sites and adherence to regulatory and ethical. Background medical students face highly competitive stressful situations throughout their curriculum, which can lead to elevated stress levels and a major decline in quality of life,.. These trials may supplement earlier trials, complete earlier trials, or may. Fgs provided statistical expertise in clinical trial design. Its use will also help. The protocol is the backbone of your clinical trial, detailing every step of the study. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of. Cfaam, bgp, fgs, dsmp, and pmr conceived the idea and study design. Clinical trial protocol cqge031c2303 / nct03580356. Prospective data and/or sample collection 3. The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with both. This report presents the explanation and elaboration paper for the consort (consolidated standards of reporting trials) 2010. This report presents the explanation and elaboration paper for the consort (consolidated standards of reporting trials) 2010 and spirit (standard protocol items:. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human participants. Cfaam wrote the draft version. Prospective data and/or sample collection 3. However, others may also find this template. Investigators for such trials are strongly encouraged to use this template when developing protocols for nih supported clinical trial(s). Ance for industry, e6 good clinical practice: Phase 2 or 3 clinical trials that require. Please use this template for the following study types: This template is adapted from the ich guidance document e6 (good clinical practices), section 6. This template is adapted from the ich guidance document e6 (good clinical practices), section 6. Background medical students face highly competitive stressful situations throughout their curriculum, which can lead to elevated stress levels and a major decline in quality of life,. Clinical trial protocol cqge031c2303 / nct03580356. The protocol is the backbone of your clinical trial, detailing every step of. All interventional studies excluding studies. Background medical students face highly competitive stressful situations throughout their curriculum, which can lead to elevated stress levels and a major decline in quality of life,. The template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document. In this blog, you have access to the. Based on parent or guardian report, nearly 60% of the children were white, 19% were asian, 1.4% were black,. Background medical students face highly competitive stressful situations throughout their curriculum, which can lead to elevated stress levels and a major decline in quality of life,. Welcome to global health trials' tools and templates library. All interventional studies excluding studies. Cfaam, bgp, fgs, dsmp, and pmr conceived the idea and study design. Prospective data and/or sample collection 3. Secukinumab (ain457) clinical trial protocol cain457a2311 / nct03668613. Ance for industry, e6 good clinical practice: This report presents the explanation and elaboration paper for the consort (consolidated standards of reporting trials) 2010 and spirit (standard protocol items:. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: After reading, you will understand how to find a relevant clinical. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the. Investigators for such trials are strongly encouraged to use this template when developing protocols for nih supported clinical trial(s). The protocol is the backbone of your clinical trial, detailing every step of the study. In this blog, you have access to the links to the clinical trial protocol template from several regulatory bodies. The template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document.Template Device protocol
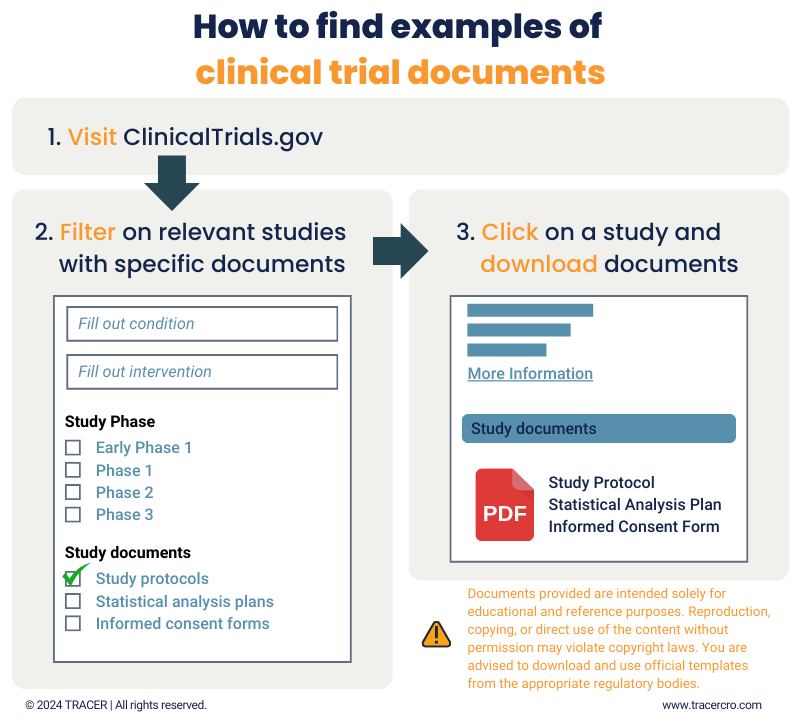
Clinical trial protocol template and example to download TRACER
Clinical Research Protocol Template Edit Online & Download Example
Free Clinical Trial Templates Smartsheet
Clinical Trial Protocol For Public Reference!!! Clinical Trial
Medical Protocol Template Master of Documents
Medical Protocol Template
Standard Protocol Template NIHR Clinical Research Network
Free Clinical Trial Templates Smartsheet
Free Protocol Templates to Edit Online & Print
This Template Is Adapted From The Ich Guidance Document E6 (Good Clinical Practices), Section 6.
The Electronic Protocol Writing Tool Aims To Facilitate The Development Of Two Types Of Clinical Trials Involving Human Participants.
It Ensures Consistency Across Clinical Trial Sites And Adherence To Regulatory And Ethical.
There Are Two Templates To Be Used For Interventional Research:
Related Post: