Eu Declaration Of Conformity Template
Eu Declaration Of Conformity Template - It may include a colour image of sufficient clarity to enable the identification of the product, where appropriate.) Eu declaration of conformity (sample) 1. The generic template of the (eu) declaration of conformity is given in annex iii of decision 768/2008/ec. Creating a declaration of conformity (doc) is an essential step for manufacturers of medical devices and in vitro diagnostic devices who wish to enter the european union market. This object is in conformity with the following union harmonisation legislation: Additional information to be mentioned on the doc may be required by. As our customers report, amazon or the german federal network agency (bundesnetzagentur) are increasingly requesting the eu declaration of conformity from. Product model/product (product, type, batch or serial number): It contains 8 points that a manufacturer must fill out to declare that a product meets relevant eu legislation and. (further) the following harmonised standards and. These products bear the ce mark indicating conformity with the provisions of these directives. How to draft the eu declaration of conformity. Standards used for demonstration of compliance: As a general requirement, the manufacturer of medical device or accessory in compliance with the applicable requirements of mdr 2017/745 must declare the conformity with an eu doc. Product model/product (product, type, batch or serial number): Pressure equipment direc˚ve (ped) 2014/68/eu. The eu declaration of conformity previously was called an ‘ec declaration of conformity’. Additional information to be mentioned on the doc may be required by. This document is an eu declaration of conformity template. As our customers report, amazon or the german federal network agency (bundesnetzagentur) are increasingly requesting the eu declaration of conformity from. It contains 8 points that a manufacturer must fill out to declare that a product meets relevant eu legislation and. It may include a colour image of sufficient clarity to enable the identification of the product, where appropriate.) The generic template of the (eu) declaration of conformity is given in annex iii of decision 768/2008/ec. Conformity assessment procedure, notified bodies,. Pressure equipment direc˚ve (ped) 2014/68/eu. Product model/product (product, type, batch or serial number): Additional information to be mentioned on the doc may be required by. How to draft the eu declaration of conformity. As our customers report, amazon or the german federal network agency (bundesnetzagentur) are increasingly requesting the eu declaration of conformity from. Standards used for demonstration of compliance: This document is an eu declaration of conformity template. As our customers report, amazon or the german federal network agency (bundesnetzagentur) are increasingly requesting the eu declaration of conformity from. It may include a colour image of sufficient clarity to enable the identification of the product, where appropriate.) The eu declaration of conformity previously. Standards used for demonstration of compliance: It may include a colour image of sufficient clarity to enable the identification of the product, where appropriate.) It contains 8 points that a manufacturer must fill out to declare that a product meets relevant eu legislation and. (further) the following harmonised standards and. Pressure equipment direc˚ve (ped) 2014/68/eu. Product model/product (product, type, batch or serial number): These products bear the ce mark indicating conformity with the provisions of these directives. This object is in conformity with the following union harmonisation legislation: Comply with medical device regulation (eu) 2017/745. It may include a colour image of sufficient clarity to enable the identification of the product, where appropriate.) Comply with medical device regulation (eu) 2017/745. Additional information to be mentioned on the doc may be required by. Standards used for demonstration of compliance: This document is an eu declaration of conformity template. In this blog you will be guided through the process of creating a fully compliant declaration of conformity (doc), so you will be able to compile. How to draft the eu declaration of conformity. Eu declaration of conformity (sample) 1. The generic template of the (eu) declaration of conformity is given in annex iii of decision 768/2008/ec. The eu declaration of conformity previously was called an ‘ec declaration of conformity’. In this blog you will be guided through the process of creating a fully compliant declaration. Creating a declaration of conformity (doc) is an essential step for manufacturers of medical devices and in vitro diagnostic devices who wish to enter the european union market. Eu declaration of conformity (sample) 1. This document is an eu declaration of conformity template. These products bear the ce mark indicating conformity with the provisions of these directives. This object is. These products bear the ce mark indicating conformity with the provisions of these directives. The generic template of the (eu) declaration of conformity is given in annex iii of decision 768/2008/ec. It contains 8 points that a manufacturer must fill out to declare that a product meets relevant eu legislation and. Essential guide to ensure compliance and meet new standards.. As a general requirement, the manufacturer of medical device or accessory in compliance with the applicable requirements of mdr 2017/745 must declare the conformity with an eu doc. It contains 8 points that a manufacturer must fill out to declare that a product meets relevant eu legislation and. How to draft the eu declaration of conformity. Conformity assessment procedure, notified. Additional information to be mentioned on the doc may be required by. Pressure equipment direc˚ve (ped) 2014/68/eu. Eu declaration of conformity (sample) 1. As a general requirement, the manufacturer of medical device or accessory in compliance with the applicable requirements of mdr 2017/745 must declare the conformity with an eu doc. As our customers report, amazon or the german federal network agency (bundesnetzagentur) are increasingly requesting the eu declaration of conformity from. The generic template of the (eu) declaration of conformity is given in annex iii of decision 768/2008/ec. The eu declaration of conformity previously was called an ‘ec declaration of conformity’. Comply with medical device regulation (eu) 2017/745. Essential guide to ensure compliance and meet new standards. Product model/product (product, type, batch or serial number): This document is an eu declaration of conformity template. It contains 8 points that a manufacturer must fill out to declare that a product meets relevant eu legislation and. These products bear the ce mark indicating conformity with the provisions of these directives. This object is in conformity with the following union harmonisation legislation: How to draft the eu declaration of conformity. Conformity assessment procedure, notified bodies, technical standards, harmonised standards, risk assessment, ce marking.Declaration Of Conformity Template
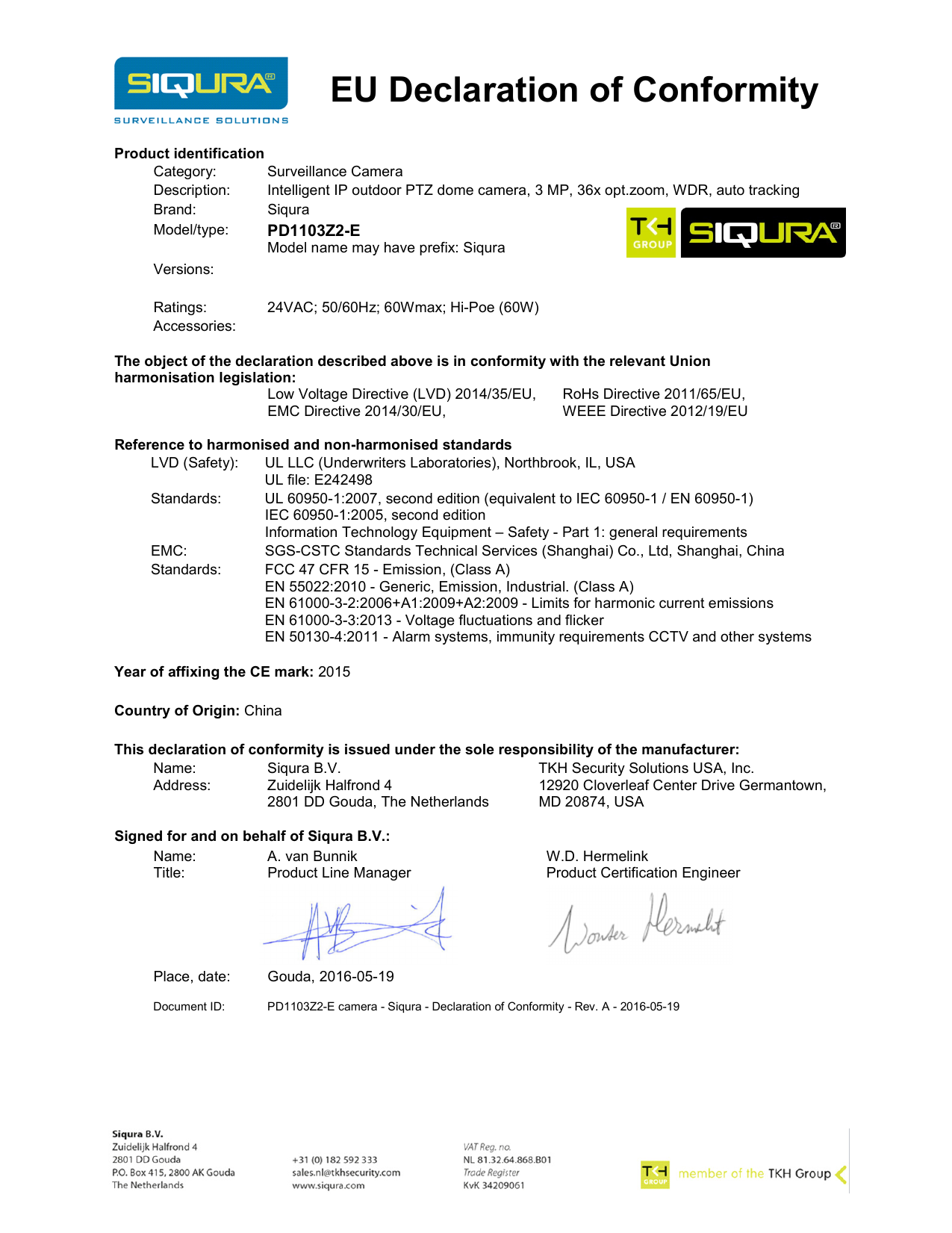
EU Declaration of Conformity
Fillable Eu Declaration Of Conformity (Doc) printable pdf download
A Guide to CE Marking EU Authorised Rep Compliance
DS80D EU DECLARATION OF CONFORMITY EN
EC Declaration of Conformity
EU Declaration of Conformity Fasetech
The EU declaration of conformity F2 Tech Notes
Eu Declaration Of Conformity Template
EU Declaration of Conformity
Creating A Declaration Of Conformity (Doc) Is An Essential Step For Manufacturers Of Medical Devices And In Vitro Diagnostic Devices Who Wish To Enter The European Union Market.
(Further) The Following Harmonised Standards And.
It May Include A Colour Image Of Sufficient Clarity To Enable The Identification Of The Product, Where Appropriate.)
In This Blog You Will Be Guided Through The Process Of Creating A Fully Compliant Declaration Of Conformity (Doc), So You Will Be Able To Compile This Document Yourself.
Related Post: