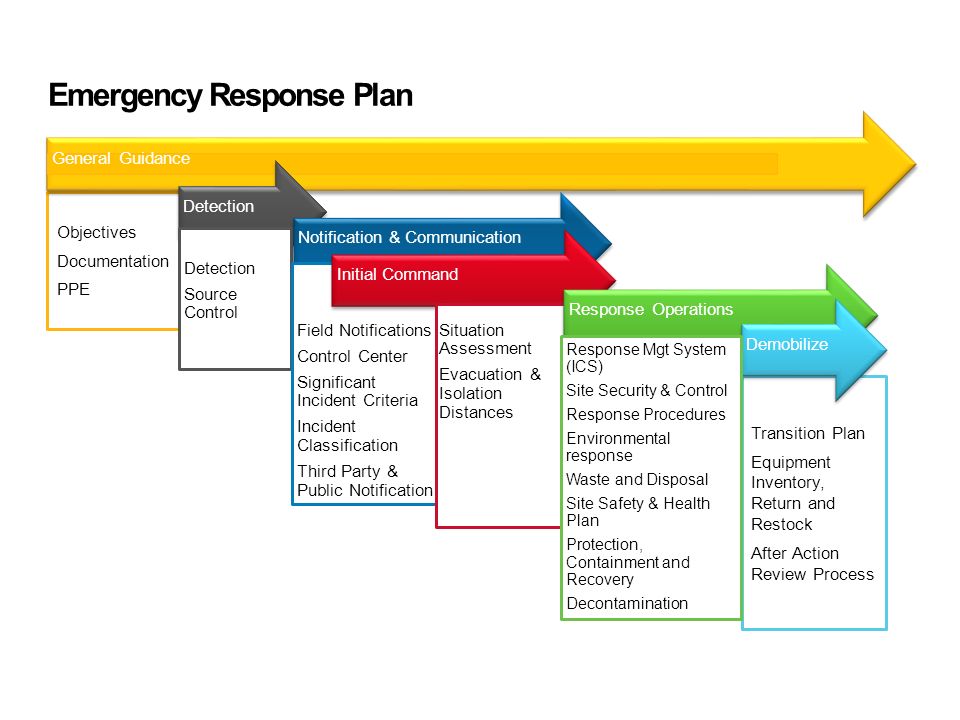
Ema Templates
Ema Templates - Ema, national competent authorities and the european commission are conducting an electronic product information (epi) pilot project to pilot use of the eu epi common standard in regulatory procedures. From 1 january 2019, the formatted letter template will not be maintained by ema and the document and references will be removed from ema corporate website. Find information for clinical trial sponsors on how to log in or register for the clinical trials information system (ctis). A number of documents in volume 10 have been revised and updated to bring them in line with the changes required by the clinical trials regulation (eu) no 536/2014. Download the main template in your languages (i keep them on my second monitor when i’m translating). This project is funded by eu4health. Read the qrd guidance documents on formatting, style and terminology. The committee for medicinal products for human use (chmp) and committee on advanced therapies (cat) should use the assessment report templates and documents listed below for the assessment of any new application in the centralised procedure. The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for product information for use by applicants and marketing authorisation holders for human medicines. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to clinical trials. Ema, national competent authorities and the european commission are conducting an electronic product information (epi) pilot project to pilot use of the eu epi common standard in regulatory procedures. From 1 january 2019, the formatted letter template will not be maintained by ema and the document and references will be removed from ema corporate website. Download the main template in your languages (i keep them on my second monitor when i’m translating). The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for product information for use by applicants and marketing authorisation holders for human medicines. The committee for medicinal products for human use (chmp) and committee on advanced therapies (cat) should use the assessment report templates and documents listed below for the assessment of any new application in the centralised procedure. Read the qrd guidance documents on formatting, style and terminology. Find information for clinical trial sponsors on how to log in or register for the clinical trials information system (ctis). Briefing document template for parallel hta coordination group (htacg)/european medicines agency (ema) joint scientific consultation (jsc) for medicinal products (mp) page contents details Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to clinical trials. This project is funded by eu4health. Download the main template in your languages (i keep them on my second monitor when i’m translating). A number of documents in volume 10 have been revised and updated to bring them in line with the changes required by the clinical trials regulation (eu) no 536/2014. Briefing document template for parallel hta coordination group (htacg)/european medicines agency (ema) joint scientific. Briefing document template for parallel hta coordination group (htacg)/european medicines agency (ema) joint scientific consultation (jsc) for medicinal products (mp) page contents details The committee for medicinal products for human use (chmp) and committee on advanced therapies (cat) should use the assessment report templates and documents listed below for the assessment of any new application in the centralised procedure. Read. Read the qrd guidance documents on formatting, style and terminology. Ema, national competent authorities and the european commission are conducting an electronic product information (epi) pilot project to pilot use of the eu epi common standard in regulatory procedures. Find information for clinical trial sponsors on how to log in or register for the clinical trials information system (ctis). Download. This project is funded by eu4health. Find information for clinical trial sponsors on how to log in or register for the clinical trials information system (ctis). Ema, national competent authorities and the european commission are conducting an electronic product information (epi) pilot project to pilot use of the eu epi common standard in regulatory procedures. The european medicines agency's (ema). This project is funded by eu4health. A number of documents in volume 10 have been revised and updated to bring them in line with the changes required by the clinical trials regulation (eu) no 536/2014. The european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and updates templates for product information for use by applicants. This project is funded by eu4health. Briefing document template for parallel hta coordination group (htacg)/european medicines agency (ema) joint scientific consultation (jsc) for medicinal products (mp) page contents details Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to clinical trials. The committee for medicinal products for human use (chmp) and. From 1 january 2019, the formatted letter template will not be maintained by ema and the document and references will be removed from ema corporate website. A number of documents in volume 10 have been revised and updated to bring them in line with the changes required by the clinical trials regulation (eu) no 536/2014. The european medicines agency's (ema). Find information for clinical trial sponsors on how to log in or register for the clinical trials information system (ctis). Read the qrd guidance documents on formatting, style and terminology. Ema, national competent authorities and the european commission are conducting an electronic product information (epi) pilot project to pilot use of the eu epi common standard in regulatory procedures. The. Ema, national competent authorities and the european commission are conducting an electronic product information (epi) pilot project to pilot use of the eu epi common standard in regulatory procedures. Read the qrd guidance documents on formatting, style and terminology. This project is funded by eu4health. Download the main template in your languages (i keep them on my second monitor when. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to clinical trials. From 1 january 2019, the formatted letter template will not be maintained by ema and the document and references will be removed from ema corporate website. This project is funded by eu4health. A number of documents in volume 10. A number of documents in volume 10 have been revised and updated to bring them in line with the changes required by the clinical trials regulation (eu) no 536/2014. Briefing document template for parallel hta coordination group (htacg)/european medicines agency (ema) joint scientific consultation (jsc) for medicinal products (mp) page contents details Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to clinical trials. The committee for medicinal products for human use (chmp) and committee on advanced therapies (cat) should use the assessment report templates and documents listed below for the assessment of any new application in the centralised procedure. Read the qrd guidance documents on formatting, style and terminology. Ema, national competent authorities and the european commission are conducting an electronic product information (epi) pilot project to pilot use of the eu epi common standard in regulatory procedures. This project is funded by eu4health. From 1 january 2019, the formatted letter template will not be maintained by ema and the document and references will be removed from ema corporate website.Ema Product Information Templates
8+ Emergency Management Plan Word Templates Free Downloads
Emergency Management Plan Template prntbl.concejomunicipaldechinu.gov.co
Emergency Management Plan 7+ Examples, Format, Pdf
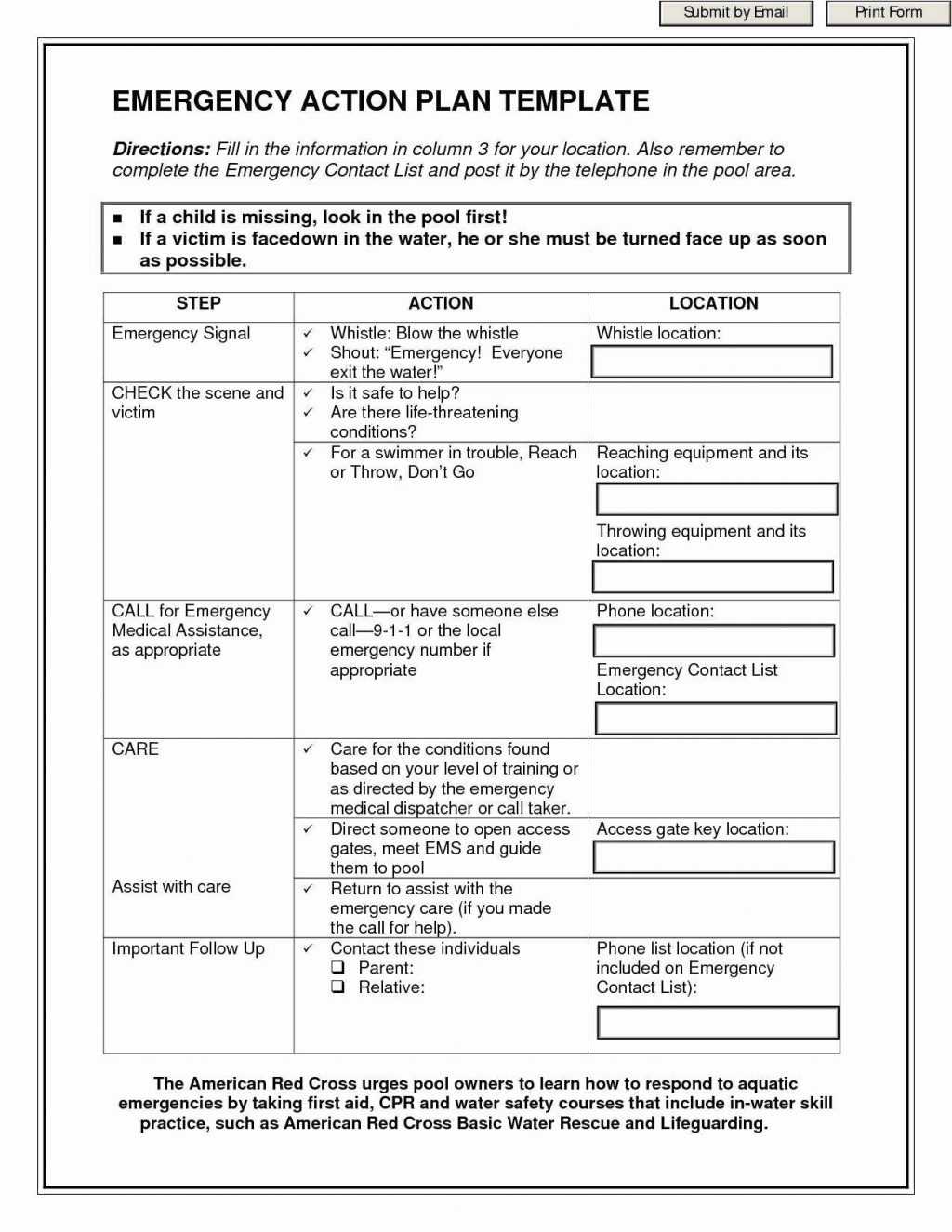
EMA Example Audit Report Template 11 January 2022 PDF Audit
Emergency Management Plan 7+ Examples, Format, Pdf
Blank Ema Template + Colouringin Dragon Cutouts Supplies For Sensei
Emergency Preparedness Plan Example Template Venngage
Ema Product Information Templates
Incident Management Plan Template
Download The Main Template In Your Languages (I Keep Them On My Second Monitor When I’m Translating).
The European Medicines Agency's (Ema) Working Group On Quality Review Of Documents (Qrd) Develops, Reviews And Updates Templates For Product Information For Use By Applicants And Marketing Authorisation Holders For Human Medicines.
Find Information For Clinical Trial Sponsors On How To Log In Or Register For The Clinical Trials Information System (Ctis).
Related Post: